
Overview
You might be wondering why Standard Operating Procedures (SOPs) are such a big deal in research. Well, let’s dive into it! SOPs are crucial for ensuring consistency, compliance, and operational efficiency. In fact, effective SOPs can cut compliance-related risks by up to 60%! Plus, they can really boost task performance, especially when you throw in some visual aids. But, it’s not all smooth sailing; there are challenges in implementing them. That’s why regular reviews and updates are so important to keep them relevant in our fast-paced research environments. So, what do you think? Ready to explore how SOPs can work for you?
Key Highlights:
- SowFlow is an intuitive platform for creating and managing SOPs, enhancing efficiency in documentation.
- Standard Operating Procedures (SOPs) are crucial for consistency and compliance in research, helping teams adhere to protocols.
- Effective SOPs can reduce compliance-related risks by up to 60% and improve operational efficiency.
- Visual SOPs significantly enhance task performance, with studies showing a 323% improvement compared to text-based guidelines.
- Resistance to change is a major challenge in SOP implementation; clear communication and training are essential.
- Regular reviews and updates of SOPs are vital to maintain relevance and compliance with current practises.
- Utilising SOP software like SowFlow streamlines documentation processes, saving time and improving productivity.
- Future trends in SOP development include the integration of AI for automated updates and mobile applications for easier access.
Introduction
You might be wondering why Standard Operating Procedures (SOPs) are such a big deal in research these days. Well, as organizations hustle to be more efficient and compliant, understanding the role of SOPs has never been more important. Think of SOPs as the backbone of research operations—they help ensure consistency and integrity across various studies and tackle those pesky regulatory requirements.
But here’s the kicker: how can managers actually put these procedures into practice in a research world that’s always changing? In this article, we’ll dive into seven key insights that shed light on what SOPs mean for research management. We’ll explore their importance, share best practices, and look at innovative tools like SowFlow that can make creating SOPs a breeze.
So, let’s get started!
SowFlow: Streamlined SOP Creation for Research Efficiency
You might be wondering how to streamline your documentation process. Well, let me introduce you to SowFlow! This intuitive platform makes it super easy for researchers like you to create and manage Standard Operating Procedures, which is what SOP means in research. With its advanced technology, you can record workflows right from your browser. How cool is that? This means you can set up your SOPs quickly and keep them up to date without the usual hassle, as SOP means in research.
Now, think about how much time you spend on documentation. SowFlow minimizes that, allowing you to focus on what really matters—your core activities. Plus, it helps ensure that everyone is on the same page, maintaining consistency in your procedures. So, if you’re looking to make your documentation process smoother and more efficient, why not give SowFlow a try? It could be just what you need!
Defining Standard Operating Procedures (SOPs) in Research
You might be wondering what SOP means in research, specifically what Standard Operating Procedures (SOPs) really are. Well, they’re detailed, documented instructions that help you perform specific tasks consistently and effectively in academic settings. Think of them as the backbone for investigators, ensuring that everyone on the team understands what SOP means in research and adheres to the same protocols. This consistency is crucial for maintaining quality and adherence in study activities.
Now, let’s talk about why SOPs are so important. They have a direct impact on compliance in studies. For instance, a biotech company that standardized its procedures managed to cut inspection observations by a whopping 50%! This really shows how effective protocols can improve regulatory standing. Plus, well-designed SOPs are vital for upholding integrity in studies. They provide clear guidance that helps prevent deviations, ensuring that what SOP means in research is adhered to for reproducible study results.
Researchers often highlight what SOP means in research as a necessity. One noted that SOP means in research, as it serves as the backbone of any solid compliance program, playing a key role in ensuring consistency and adherence to regulatory requirements. Interestingly, studies reveal that about 85% of failures in investigations come from deficiencies in systems and processes, not individual errors. So, investing in strong SOPs not only boosts operational efficiency but also safeguards data integrity and ethical standards in scientific practices.
In fast-paced research environments, clarity and accessibility of SOPs can really boost productivity. Have you heard about visual SOPs? They’ve been shown to enhance task performance by 323% compared to traditional text-based guidelines! This just goes to show how visual aids can simplify complex processes. By incorporating visual elements, SOPs foster a culture of reliability and consistency, benefiting both researchers and participants alike.
Speaking of innovation, let’s dive into SowFlow. This platform is a game changer, allowing teams to create SOPs and training materials without the hassle of taking separate screenshots or leaving the browser. With its user-friendly interface and integrated tools, creating SOPs becomes a breeze. As Anastasia Masadi, a Product Owner, put it, "SowFlow gave me time from my life back." That’s a testament to how this platform boosts efficiency in the SOP creation process. To really get the most out of your SOPs, remember that regular reviews and updates are crucial to keep them relevant and aligned with the latest best practices.
Importance of SOPs for Consistency and Compliance in Research
You might be wondering what SOP means in research and why it is such a big deal in experimental settings. Well, they’re crucial for ensuring everyone’s on the same page and sticking to the plan. By standardizing procedures, understanding what SOP means in research can significantly cut down on variability in study outcomes, which is key to getting reliable results. Research groups that follow these established protocols not only check off regulatory boxes but also uphold ethical standards, boosting the credibility of their findings.
Speaking of benefits, did you know that effective SOPs can lead to a whopping 60% drop in compliance-related risks? That’s according to Deloitte, and it means your investigative practices can align nicely with Good Clinical Practice (GCP) guidelines. Plus, organizations with clear SOPs tend to outperform their competitors by 31%. That’s a solid competitive edge! For instance, one biotech company that standardized its clinical research procedures saw a 50% reduction in inspection observations. Talk about tangible benefits from sticking to the plan!
And it doesn’t stop there—companies with effective SOPs report up to 25% lower turnover rates. That really highlights the value of these practices. To make the most of your SOPs, it’s a good idea for operations managers to set up a regular review schedule. This way, you can keep them relevant and compliant. In summary, knowing what SOP means in research is essential for promoting consistency, boosting compliance, and ultimately improving the integrity of your results. So, what’s your next step in refining your own procedures?
Key Components of Effective SOPs for Research
Effective Standard Operating Procedures (SOP means in research) focus on clarity and user-friendliness. You might be wondering what essential elements really make an SOP shine. Let’s break it down:
- Clear Title: Start with a concise title that truly reflects what the SOP is about.
- Purpose: A brief statement that defines the intent of the SOP—this helps guide users on why it’s relevant to them.
- Scope: This section clarifies who or what the procedures apply to, including any exclusions.
- Definitions: Clear definitions of terms and acronyms can really help users understand the content without any confusion.
- Detailed Procedures: Step-by-step instructions outline the necessary actions, ensuring tasks are performed consistently and correctly.
- Roles and Responsibilities: Clearly defined roles help everyone understand who’s accountable for each part of the process.
- Revision History: This is crucial for tracking changes and updates, ensuring the SOP stays current. Regular updates are key, especially when you notice rising error rates or frequent questions from employees about processes. With SowFlow, teams can instantly update and revise their documentation, using features like real-time collaboration and version control to keep SOPs relevant in our ever-changing business world.
- Approval Signatures: Including signatures from relevant roles, like authors and reviewers, ensures the SOP has been validated and meets organizational standards.
Incorporating these elements not only boosts the effectiveness of your SOPs but also fosters compliance and operational efficiency. Research shows that what SOP means in research typically involves effective documents ranging from 2 to 8 pages, focusing on clarity over length, which is vital for keeping users engaged and understanding the material. By prioritizing these components and utilizing SowFlow's instant documentation solution, organizations can create detailed operational procedures that act as reliable guides in experimental environments. This empowers teams with efficient workflow sharing and knowledge management.
So, how can you implement effective SOPs? Consider conducting regular reviews and involving team members in the update process to ensure everything stays relevant and accurate. Now, let’s dive into how you can make your documentation even better!
Challenges in Developing SOPs for Research Environments
You might be wondering why developing Standard Operating Procedures (SOPs) in research environments can feel like navigating a minefield, and what SOP means in research. Well, a big part of it is the resistance to change from staff. Did you know that a whopping 71% of employees feel overwhelmed by the level of change at work? That’s a lot of folks who might hesitate to accept new procedures. And it gets even more interesting: 86% of employees aged 16 to 24 report feeling overwhelmed by workplace changes, which shows just how impactful change fatigue can be for younger employees. Plus, a study found that 29% of employees think organizational change isn’t communicated clearly, leading to confusion and reluctance to embrace new procedures.
So, how can organizations tackle these challenges? First off, clear communication is key. Involving stakeholders in the process of understanding what SOP means in research can really make a difference. When group members are part of the conversation about why standard operating procedures are important, it fosters a sense of ownership and can reduce pushback. For example, organizations that take an employee-driven approach to change can implement new procedures about 33% faster than those that stick to a top-down strategy. That really highlights the need to bridge the gap between leaders and employees when it comes to change strategies.
Now, let’s talk about training. It’s crucial to ensure that all team members are well-versed in the standard operating procedures. Comprehensive training programs should not only cover the specific procedures but also clarify what SOP means in research. By providing ongoing updates and feedback on SOP implementation, organizations can keep everyone in the loop and engaged, leading to a smoother transition and better adherence to the new protocols.
In summary, understanding what SOP means in research is crucial for overcoming resistance to its implementation, which requires a multifaceted approach that emphasizes communication, stakeholder involvement, and continuous training. As Dr. Jeremy Pollack points out, without effective change management consulting and implementation planning, even the best intentions can lead to transformation failure. By focusing on these areas, organizations can boost the effectiveness of their standard operating procedures and ensure they stay relevant and current.
Best Practices for Writing Clear and Effective SOPs
You might be wondering how to develop clear and effective Standard Operating Procedures (SOPs), and what SOP means in research. Well, it’s all about using simple language and steering clear of jargon to make sure everyone can access the information. When you structure the document logically, it really helps users follow the procedures with ease. Plus, incorporating visual aids like flowcharts and checklists can significantly boost understanding. Research even shows that people perform tasks 323% better when they have visuals to guide them instead of just written instructions.
Speaking of visuals, flowcharts can simplify complex processes, which makes them perfect for decision-making tasks. And checklists? They ensure thoroughness without overwhelming you with too much detail. Now, here’s a thought: involving your team in the SOP writing process not only reflects real-world practices but also fosters greater buy-in and adherence.
Regularly reviewing and updating your SOPs is crucial to keep them relevant and effective. After all, outdated practices can lead to compliance violations and operational hiccups. By prioritizing clarity and visual engagement, you can really enhance the effectiveness of your standard operating procedures, as understanding what SOP means in research is crucial. Ultimately, this leads to improved operational efficiency, which is something every organization strives for!
Training Strategies for Effective SOP Implementation
You might be wondering how to ensure successful SOP implementation in your organization. Well, one key approach is to adopt effective training strategies that include:
- Regular training sessions
- Hands-on practice
- A mix of formats like workshops and e-learning modules
An essential part of this method? Creating a feedback loop! This allows employees to share their experiences and suggest improvements to the standard operating procedures. Not only does this foster a culture of continuous learning, but it also boosts employee engagement and ownership of the processes.
Take Starbucks, for example. They’ve nailed it with detailed SOPs that ensure consistent service across all locations, showing just how impactful structured training can be on operational efficiency. Plus, research shows that organizations with strong SOP training programs enjoy significantly higher compliance rates, which means fewer errors and a boost in overall productivity. As Manasi Nair puts it, "SOP training isn’t just about following instructions—it’s the foundation for consistency, efficiency, and compliance across industries."
So, by prioritizing hands-on training and actively seeking feedback, businesses can build a more adaptable and effective SOP framework that meets the evolving needs of their teams. And don't forget, connecting SOP training directly to employees' daily responsibilities and providing ongoing support are crucial for keeping that training effective and ensuring that employees don’t slip back into old habits. Now, let’s dive into how you can implement these strategies in your own organization!
Importance of Regular SOP Review and Updates
You might be wondering why regular reviews and updates of Standard Operating Procedures (SOPs) are so important, as understanding what SOP means in research is crucial. Well, keeping them aligned with current practices and regulatory requirements is essential! Creating a structured review timetable—whether yearly, semi-annually, or quarterly—ensures that your SOPs remain relevant and effective. For example, organizations that opt for quarterly reviews can quickly adapt to changes in market conditions and operational demands, boosting their agility and compliance.
Engaging everyone in the review process not only brings in valuable insights but also helps foster a sense of ownership and accountability towards these procedures. Involving those frontline workers, who are often facing changing challenges, can lead to more practical and effective procedures that truly meet operational needs. Plus, leveraging technology for document management can really streamline the review process, ensuring timely updates and reducing the risk of outdated practices.
So, by prioritizing regular SOP means in research reviews, organizations can ensure they uphold high standards of quality, safety, and efficiency. This, in turn, drives continuous improvement and operational excellence. Now, let’s dive into how you can implement these reviews in your organization!
Utilizing SOP Software for Enhanced Documentation Efficiency
You might be wondering how to make your documentation process smoother and more efficient. Well, utilizing SOP software can really boost your documentation game! With customizable templates, automation features, and centralized access to standard operating procedures, tools like SowFlow make it a breeze for teams to create, update, and manage what SOP means in research. This not only lightens the administrative load but also ensures that everyone has quick access to the latest procedures.
Now, let’s dive into why this matters. This streamlined approach saves you valuable time and clarifies what SOP means in research, aiding in the improvement of compliance and consistency across all your study activities. Remember what W. Edwards Deming said about ongoing enhancement being vital for survival? By cutting down the time spent on documentation, your team can focus more on what really matters—boosting productivity and sparking innovation.
And speaking of costs, as Taiichi Ohno wisely noted, "Costs do not exist to be calculated. Costs exist to be reduced." By embracing efficient SOP software like SowFlow, you can avoid amplifying inefficiencies, making sure your documentation processes are not just effective but also cost-efficient. So, why not explore these solutions and see how they can transform your workflow?
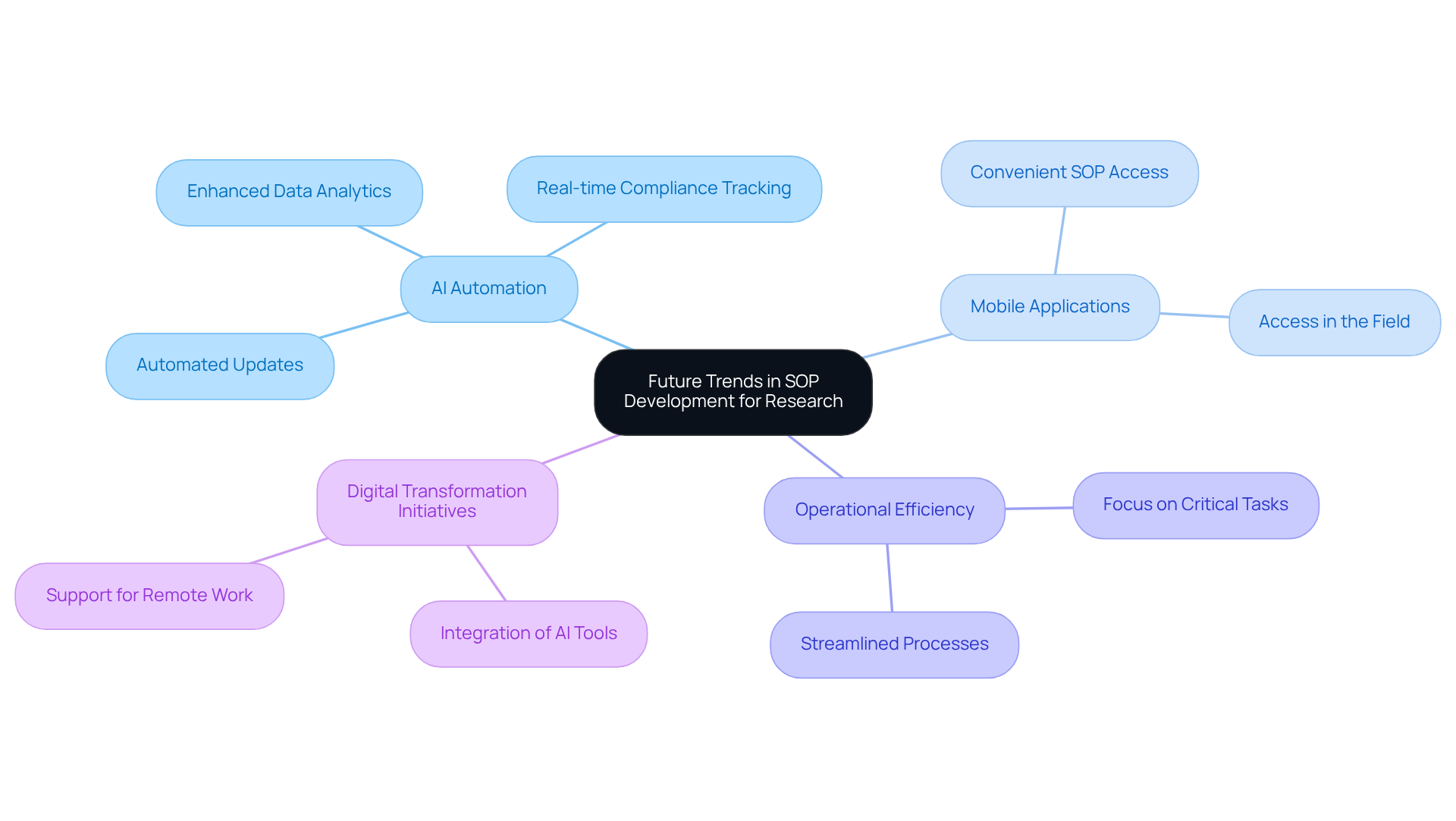
Future Trends in SOP Development for Research
You might be wondering how future trends in SOP development are shaping up in research, particularly in understanding what SOP means in research, especially with artificial intelligence stepping into the spotlight. Well, AI is set to revolutionize how organizations manage and update their procedures. Imagine automated updates ensuring that your standard operating procedures are always up-to-date and in line with those ever-changing regulatory standards. Plus, with enhanced data analytics powered by AI, organizations can track compliance more effectively, giving you insights that can inform necessary adjustments in real-time.
Now, let’s dive into another exciting aspect: mobile applications! These will allow researchers to access standard operating procedures conveniently in the field, which is what SOP means in research, making it easier to stick to established protocols. As research environments become more dynamic, understanding what SOP means in research will be crucial for keeping your SOPs effective and efficient. This not only boosts operational efficiency but also helps reduce the risk of non-compliance.
Speaking of streamlining processes, the adoption of AI in SOP management empowers teams to focus on what really matters—critical tasks. This fosters a culture of continuous improvement and innovation. And let’s not forget the increasing demand for digital transformation initiatives and efficient workflow management. It underscores the necessity of adopting AI tools like Botable, which can assist with everything from simple FAQs to complex inquiries.
With the global SOP software market projected to hit around USD 2.7 billion by 2032, integrating AI in SOP management isn’t just a nice-to-have; it’s essential for organizations looking to stay ahead in this competitive landscape. So, what do you think? Are you ready to explore these exciting advancements in SOP development?
Conclusion
You might be wondering what SOP really means in the world of research, right? Well, understanding this is super important for managers who want to boost their team's efficiency and stay compliant. Standard Operating Procedures (SOPs) are like a roadmap, guiding researchers through consistent and effective practices. This not only leads to high-quality results but also keeps everyone on the right side of regulatory standards.
Throughout the article, we highlighted just how crucial SOPs are for fostering consistency, compliance, and integrity in research processes. We even showcased platforms like SowFlow, which can make creating SOPs a breeze. Imagine how much easier documentation and team collaboration can be with the right tech! Plus, we touched on the need for regular reviews and updates, effective training strategies, and the common hurdles organizations face when developing and implementing SOPs.
So, in conclusion, embracing effective SOP practices isn’t just a box to check off; it’s a strategic move for research managers. By welcoming innovative solutions and nurturing a culture of continuous improvement, you can really ramp up productivity and compliance. As the research landscape keeps changing, staying in the loop about best practices and new trends in SOP development will be key for organizations looking to thrive in a competitive environment. Let’s keep the conversation going about how to make this all work for you!
Frequently Asked Questions
What is SowFlow?
SowFlow is an intuitive platform designed to help researchers create and manage Standard Operating Procedures (SOPs) efficiently. It allows users to record workflows directly from their browser, making the documentation process quicker and easier.
What does SOP stand for in research?
SOP stands for Standard Operating Procedures. These are detailed, documented instructions that guide researchers in performing specific tasks consistently and effectively in academic settings.
Why are SOPs important in research?
SOPs are crucial for ensuring consistency and compliance in research activities. They help maintain quality, adhere to regulatory requirements, and prevent deviations that could affect the integrity of study results.
How do SOPs impact compliance in studies?
Well-designed SOPs can significantly improve compliance, as evidenced by a biotech company that reduced inspection observations by 50% after standardizing its procedures. This highlights the effectiveness of SOPs in enhancing regulatory standing.
What are the benefits of visual SOPs?
Visual SOPs have been shown to enhance task performance by 323% compared to traditional text-based guidelines. Incorporating visual elements simplifies complex processes and fosters a culture of reliability and consistency.
How can SowFlow improve the SOP creation process?
SowFlow streamlines the SOP creation process by allowing teams to create SOPs and training materials without needing to take separate screenshots or leave the browser. Its user-friendly interface makes the process efficient and less time-consuming.
What is the relationship between SOPs and research outcomes?
Standardizing procedures through SOPs significantly reduces variability in study outcomes, which is essential for obtaining reliable results. Following established protocols also helps uphold ethical standards and boosts the credibility of findings.
How do effective SOPs affect organizational performance?
Organizations with effective SOPs can experience up to a 60% drop in compliance-related risks and outperform competitors by 31%. Additionally, they may report lower turnover rates, with some companies seeing a reduction of up to 25%.
What is recommended for maintaining effective SOPs?
It is advisable for operations managers to establish a regular review schedule for SOPs to ensure they remain relevant and compliant with the latest best practices. Regular updates help keep procedures aligned with current standards.
👍
What others are liking
5 Steps to outline your ideal documentation structure
5 MINS READ
Where to start the your journey of mapping out your ideal documentation structure, aligning it with the very heartbeat of your organization?
Defining a winning level of detail in your process
3 MINS READ
What is too much detail, and what is too little? This article described in that winning level detail about what detail is enough.





